Crystallization can occur along different paths depending on the ratio of substances in a melt as well as the temperature and pressure conditions from which cooling began.
Here is the phase diagram from that Wikipedia article:
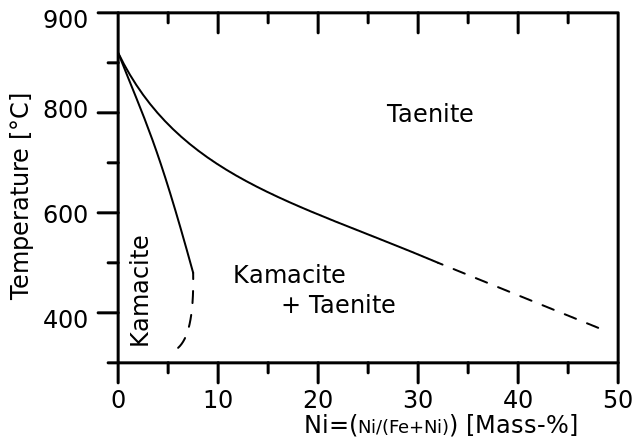
The Staunton meteorite includes kamacite with taenite, and the iron includes 8.6% nickel. So if you follow the vertical line that sits at 8.6% on the phase diagram you can see that the phase change occurs about 700˚C; it's actually 723 ˚C.
So it's not until the meteor has cooled below 1000˚C that kamacite can form at all. But the melting point of the iron-nickel alloy is around 1500˚C, roughly the melting point of iron. So this is not crystallisation from a melt, but a painfully slow recrystallisation by diffusion of atoms through a
solid metallic lattice.
For the largest crystal the meteorite has to stay as high as possible below that 723˚C for millions of year, because the cooler it gets the slower the diffusion happens, so that just a few hundred degrees lower the recrystallisation process essentially stops, leaving the crystals at a particular size.
Stuu: Wherever these meteorites came from, it wasn't from the earth.
Apart from meteorites, and some native iron deposits in Greenland that resulted from reduction of metal oxides due to carbon-laden magma or lava, iron-nickel alloys are not found in the crust, but in the inner and outer core below the mantle. This is because materials were sorted by density during the accretion that formed the earth.
Presumably you think they cooled over millions of years inside another planet and then they escaped that planet's gravity.
Planetesimals, not planets, and they are released through collisions with other planetesimals. Although, there is no particular problem with escaping gravity: rocks from the surface of Mars have made it to earth after being ejected in a collision with an asteroid or comet.
Stuart